
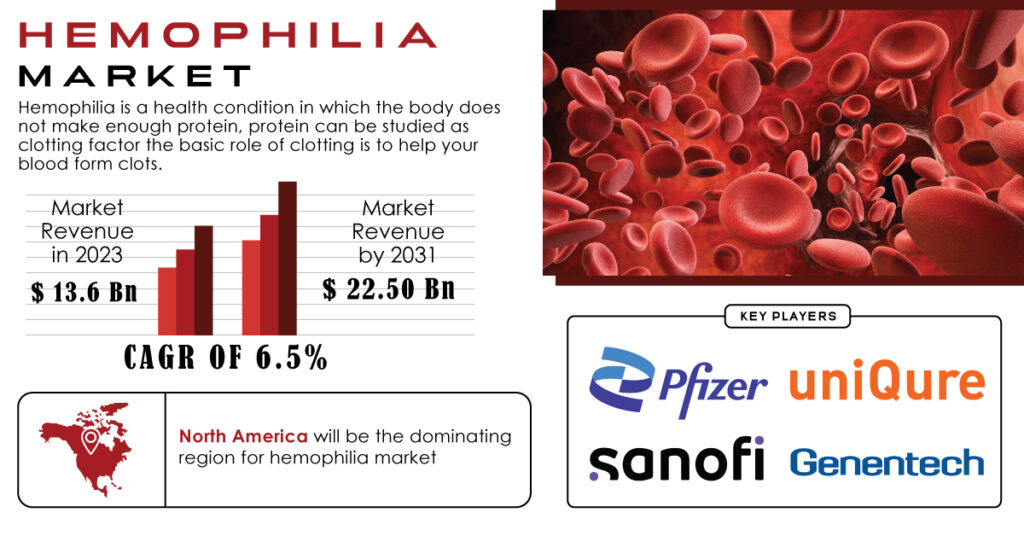
The Hemophilia Market was valued at USD 13.6 Billion in 2023 and is anticipated to reach USD 22.50 Billion by 2031. This translates to a remarkable compound annual growth rate (CAGR) of 6.5% throughout the forecast period.
The World Federation of Hemophilia estimates that 386,966 people globally have bleeding disorders, with hemophilia accounting for 233,577 cases. This high prevalence, coupled with increasing government initiatives to improve access to treatment, is fueling market growth.
Furthermore, rising research activity is bolstering the market. For instance, the National Heart, Lung, and Blood Institute awarded USD 2 million to a researcher at Indiana University School of Medicine in March 2022 for developing a gene therapy approach for hemophilia. Additionally, the US Food and Drug Administration (FDA) greenlit Freeline Therapeutics’ Phase 1/2 B-LIEVE clinical trial of FLT180a for treating hemophilia B in March 2022. These advancements highlight the continuous innovation in the hemophilia treatment landscape, which is expected to propel market growth.
Download Free Sample Report of Hemophilia Market @ https://www.snsinsider.com/sample-request/2705
List of Hemophilia Companies Profiled in Report:
- akeda Pharmaceutical Company Limited
- Alnylam Pharmaceuticals
- Baxalta
- Baxter International Inc.
- Bayer AG
- Biogen
- BioMarin Pharmaceutical Inc.
- CSL Behring
- Hoffmann La-Roche Ltd.
- Genentech USA Inc.
- Grifols S.A.
- Kedrion S.p.A.
- Novo Nordisk A/S
- Octapharma AG
- Pfizer Inc.
- Sanofi S.A.
- Spark Therapeutics, Inc.
- Takeda Pharmaceutical Company Limited
- uniQure N.V.
(To view Full list of companies, Ask for Sample Report)
Innovation Driving Growth
The hemophilia market is flourishing due to several key drivers. Firstly, the rising prevalence of hemophilia globally necessitates effective treatment options. Secondly, there is a significant increase in research and development activities by pharmaceutical companies, leading to a robust pipeline of novel therapeutics. For instance, in April 2020, the FDA approved Sevenfact (coagulation factor VIIa [recombinant] -jncw), a genetically engineered product for treating bleeding episodes in adolescents and adults with hemophilia. Such product approvals are anticipated to propel market growth.
Furthermore, increasing financial support for R&D through investments and grants is boosting market growth. In March 2022, the Indiana University School of Medicine received USD 12 million from the National Heart, Blood, and Lung Institute to develop improved hemophilia therapies. This exemplifies the growing focus on R&D, paving the way for advanced treatment approaches like gene therapy and monoclonal antibodies.
In February 2023, The US Food and Drug Administration (FDA) approved ALTUVIIIO (Antihemophilic Factor (Recombinant), Fc-VWF-XTEN Fusion Protein-ehtl), previously known as efanesoctocog alfa. This first-in-class, high-sustained factor VIII replacement therapy offers a significant breakthrough. ALTUVIIIO is the first and only hemophilia A treatment to deliver normal to near-normal factor activity levels (over 40%) for most of the week with once-weekly dosing. This significantly reduces bleeding episodes compared to prior factor VIII prophylaxis.
In January 2023, BioMarin Pharmaceutical Inc., a leader in gene therapy, released positive long-term data from their ongoing global Phase 3 GENEr8-1 study of ROCTAVIANTM (valoctocogene roxaparvovec). This investigational one-time gene therapy for treating adults with severe hemophilia A is the largest and longest global Phase 3 study for any gene therapy in hemophilia, with 134 participants. The positive results from this extended follow-up period bolster the potential of gene therapy as a transformative treatment option for hemophilia A.
Significant Market Share Is Expected to Be Held by Hemophilia A
Hemophilia A, the most common severe bleeding disorder, is expected to hold a significant market share due to factors such as a rising prevalence of cases, increased awareness programs, and a surge in different therapies for Hemophilia A. According to the World Federation of Hemophilia Report on the Annual Global Survey 2021, 185,318 cases of hemophilia A were identified globally in 2021, highlighting the significant patient population driving market growth in this segment.
Furthermore, product approvals like the European Commission’s (EC) conditional marketing authorization granted to valoctocogene roxaparvovec gene therapy (ROCTAVIAN) in August 2022 and the FDA’s Breakthrough Therapy designation for efanesoctocog alfa (BIVV001) in June 2022 are propelling market growth. Additionally, research advancements like in vivo genome-editing programs based on CRISPR/Cas9 are being explored for hemophilia A treatment, offering promising possibilities for the future.
Treatment Type: Replacement Therapy Dominates
Replacement therapy, the mainstay of hemophilia treatment, currently dominates the market. This therapy involves providing the missing clotting factor (factor VIII for hemophilia A and factor IX for hemophilia B) to control and prevent bleeding episodes. However, other treatment types like gene therapy and factor VIII prophylaxis are gaining traction due to their potential for long-term benefits and reduced bleeding frequency.
Hemophilia Market
Have Any Query? Ask Our Experts @ https://www.snsinsider.com/enquiry/2705
North America Dominates with Innovation
North America is expected to hold a significant share in the hemophilia market and maintain its dominance throughout the forecast period.
North America has a high prevalence of hemophilia, creating a substantial demand for effective treatment options. North America boasts a well-developed research infrastructure with significant government and private funding allocated to hemophilia research. This fosters a dynamic environment for scientific breakthroughs and the development of novel therapies.
Regulatory bodies in North America, such as the US Food and Drug Administration (FDA), have a well-established framework for drug approvals. This streamlined process facilitates the timely introduction of new hemophilia treatments to the market.
For instance, according to the CDC update in August 2022, about 33,000 people in the US contain hemophilia. Additionally, studies like the one published in Frontiers in Immunology in April 2021 highlight the successful application of Adeno-associated virus (AAV)-mediated gene transfer for hemophilia B in the United States, paving the way for wider adoption of gene therapy in the region. Furthermore, rising initiatives from key market players, such as BioMarin Pharmaceutical Inc.‘s positive results from their Phase 3 GENEr8-1 study analysis on valoctocogene roxaparvovec presented in February 2022, and approvals like Novo Nordisk receiving Health Canada approval for REBINYN for routine prophylaxis in hemophilia B patients in October 2022, are expected to propel market growth in North America.
Key Takeaways from the Hemophilia Market Study
- A detailed market sizing and growth forecast for the global hemophilia market over the next seven years.
- In-depth analysis of the key market drivers, restraints, and trends shaping the hemophilia market landscape.
- A thorough exploration of the market segmentation by disease type, treatment type, and region.
- Identification of promising areas for future growth within the hemophilia market.
- Expert insights on the impact of current and future technological advancements on hemophilia treatment strategies.
Table of Content
Chapter 1 Introduction
Chapter 2 Research Methodology
Chapter 3 Hemophilia Market Dynamics
Chapter 4 Impact Analysis (COVID-19, Ukraine- Russia war, Ongoing Recession on Major Economies)
Chapter 5 Value Chain Analysis
Chapter 6 Porter’s 5 forces model
Chapter 7 PEST Analysis
Chapter 8 Hemophilia Market Segmentation, By Therapy Type
- Replacement Therapy
- Clotting Factors
- Plasma-derived factor concentrate
- Recombinant factor concentrate
- Medications
- Hemlibra
- DDAVP/Stimate
- Amicar
- Fibrin Sealants
- Others
- Physical Therapy
- Immune Tolerance Induction (ITI) Therapy
- Vaccination
- Gene Therapy
- Others
Chapter 9 Hemophilia Market Segmentation, By Indications
- Type A
- Type B
- Type C
- Type D
Chapter 10 Regional Analysis
Chapter 11 Company profile
Chapter 12 Competitive Landscape
Chapter 13 Use Case and Best Practices
Chapter 14 Conclusion
Continued…
Purchase Hemophilia Market Report @ https://www.snsinsider.com/checkout/2705
About US:
SNS Insider has been a leader in data and analytics globally with its authentic consumer and market insights. The trust of our clients and business partners has always been at the center of who we are as a company. We are a business that leads the industry in innovation, and to support the success of our clients, our highly skilled engineers, consultants, and data scientists have consistently pushed the limits of the industry with innovative methodology and measuring technologies.
Contact Us:
Akash Anand – Head of Business Development & Strategy
Phone: +1-415-230-0044 (US) | +91-7798602273 (IND)


