
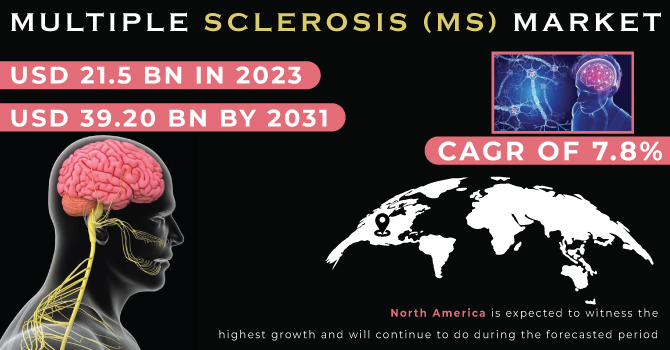
The Multiple Sclerosis (MS) Market, valued at USD 21.5 Billion in 2023, is anticipated to reach a staggering USD 39.20 Billion by 2031. This translates to a remarkable compound annual growth rate (CAGR) of 7.8% throughout the forecast period from 2024 to 2031.
Multiple Sclerosis (MS) Market
The Multiple Sclerosis (MS) market is projected to experience significant growth in the coming years, fueled by the increasing prevalence of MS, growing investments in research and development (R&D) for novel therapeutics, and a surge in product launches by key players in the market.
Download Free Sample Report of Multiple Sclerosis (MS) Market @ https://www.snsinsider.com/sample-request/2706
List of Multiple Sclerosis (MS) Companies Profiled in Report:
- Abbott
- AbbVie Inc
- Actelion Pharmaceuticals Ltd
- Allergan
- AstraZeneca
- Bayer AG
- Berlex
- Biogen
- Cipla Inc
- Reddy’s
- Eli Lilly and Company
- Hoffmann-La Roche Ltd
- Genentech Inc.
- GlaxoSmithKline plc
- Johnson & Johnson
- Merck KGaA
- Mylan N.V.
- Novartis AG
- Opexa Therapeutics Inc.
- Pfizer Inc
- Sanofi
- Teva Neuroscience Inc.
- Zydus Cadila
(To view Full list of companies, Ask for Sample Report)
Multiple Sclerosis is an autoimmune disease that disrupts the central nervous system. It is characterized by inflammation, demyelination (damage to the protective sheath around nerve fibers), and neurodegeneration. The number of people diagnosed with MS globally has been steadily rising, with the Multiple Sclerosis International Federation reporting an increase from 2.3 million in 2013 to 2.8 million in 2020. This growing prevalence of relapsing-remitting MS and primary-progressive MS has fueled the demand for effective diagnosis and treatment options worldwide. Governments are actively supporting research and development of MS therapies in response to this growing public health concern.
Innovation Driving Growth
The MS market is expected to witness a rapid growth trajectory due to several key drivers. Firstly, the rising prevalence of MS, as discussed earlier, creates a significant demand for effective treatments. Secondly, there is a significant increase in research and development activities by pharmaceutical companies, leading to a robust pipeline of novel therapeutics. For instance, according to National Multiple Sclerosis Society UK Report published in May 2022, over 130,000 people in the United Kingdom alone are living with MS, with around 7,000 new diagnoses every year. This growing burden of MS is projected to create lucrative growth opportunities for the market.
Furthermore, key pharmaceutical companies are investing heavily in R&D to expand their MS treatment portfolios. These companies are aiming to target a wider range of MS indications, catering to a larger patient population. In a recent example, Tiziana Life Sciences received FDA approval in October 2023 for their drug candidate foralumab, a self-administered intranasal treatment for MS. Additionally, extended applications for existing drugs are also boosting market growth. For instance, Teva Pharmaceuticals Europe BV obtained approval from the European Union (EU) health authorities in February 2022 for COPAXONE (Glatiramer Acetate (GA) injection) to be used by breastfeeding mothers. This highlights the focus on expanding treatment options and improving patient convenience.
The market is further fueled by a rise in research funding, grants, and other investments dedicated to MS research. For instance, scientists at Menzies School of Health Research received grants totaling USD 818,966 in February 2023 to support their MS Research Flagship program, which aims to minimize the impact of MS on individuals and communities.
Oral Route of Administration Leads the Market
The oral route of administration segment is poised for significant growth within the MS market. This preference stems from the advantages of improved patient satisfaction and increased therapeutic compliance compared to other routes, such as injections. Additionally, a growing number of product launches and approvals for oral MS medications, such as tablets and capsules, are further propelling segment expansion. Amneal Pharmaceuticals, Inc. launched LYVISPAH in June 2022, a US FDA-approved medication for managing spasticity related to MS and other spinal cord disorders. Similarly, Sandoz expanded its MS portfolio with the launch of Dimethyl fumarate HEXAL, a cost-effective generic treatment for relapsing-remitting MS (RRMS) in Germany, in June 2022. These developments, along with a rising pipeline of oral MS therapeutics, are expected to solidify the dominance of the oral route of administration segment.
North America Dominates with Innovation at the Forefront
North America is expected to hold a significant share in the Multiple Sclerosis (MS) market and maintain its dominance throughout the forecast period.
Several leading pharmaceutical and biotechnology companies with robust MS treatment pipelines are headquartered in North America. This concentration of expertise and resources fuels innovation and product development in the region.
North America boasts a well-developed research infrastructure with significant government and private funding allocated to MS research. This fosters a dynamic environment for scientific breakthroughs and the development of novel therapies.
Regulatory bodies in North America, such as the US Food and Drug Administration (FDA), have a well-established framework for drug approvals. This streamlined process facilitates the timely introduction of new MS treatments to the market.
For instance, according to the Multiple Sclerosis Association of America’s May 2022 update, nearly one million individuals in the United States alone are living with MS. Furthermore, the presence of key market players, such as TG Therapeutics, which received US FDA approval for BRIUMVI (ublituximab-xiiy) in December 2022 for treating relapsing forms of MS, further underscores North America’s prominent position in the MS market. Additionally, the surge in research grants and supportive legislation, like the National Multiple Sclerosis Society’s USD 4.4 million investment in new research projects in November 2023, is propelling further market growth in the region. Collaboration between drug companies and governments, exemplified by Novartis Pharmaceuticals Canada Inc.’s efforts to widen access to Kesimota (ofatumumab) for relapsing-remitting MS patients in Ontario and Quebec, is another factor driving regional market expansion.
Have Any Query? Ask Our Experts @ https://www.snsinsider.com/enquiry/2706
Recent Developments in the Multiple Sclerosis Market
- August 2023: The U.S. Food and Drug Administration (FDA) approved the first biosimilar drug, Tyryko (natalizumab), for treating relapsing forms of multiple sclerosis (MS). This approval offers patients a more affordable treatment option compared to the brand-name drug, Tysabri. Biosimilars are highly similar versions of existing biological drugs and can play a significant role in increasing access to expensive medications.
- October 2023: EMD Serono, a subsidiary of Merck KGaA, Darmstadt, Germany, announced positive results from the Phase III DELIVER-MS study evaluating cladribine tablets for the treatment of adults with relapsing forms of MS. The study met its primary endpoint of demonstrating a statistically significant reduction in annualized relapse rate (ARR) compared to placebo.
Key Takeaways from the Multiple Sclerosis (MS) Market Study:
- By purchasing this comprehensive report on the Multiple Sclerosis (MS) Market, you gain valuable insights into:
- A detailed market sizing and growth forecast for the global MS market over the next seven years.
- In-depth analysis of the key market drivers, restraints, and trends shaping the MS market landscape.
- A thorough exploration of the market segmentation by route of administration, drug type, and disease type.
- Identification of promising regional markets and emerging players in the MS treatment landscape.
- Expert insights on the impact of current and future technological advancements on MS treatment strategies.
Table of Content
Chapter 1 Introduction
Chapter 2 Research Methodology
Chapter 3 Multiple Sclerosis (MS) Market Dynamics
Chapter 4 Impact Analysis (COVID-19, Ukraine- Russia war, Ongoing Recession on Major Economies)
Chapter 5 Value Chain Analysis
Chapter 6 Porter’s 5 forces model
Chapter 7 PEST Analysis
Chapter 8 Multiple Sclerosis (MS) Market Segmentation, By Drug Class
Chapter 9 Multiple Sclerosis (MS) Market Segmentation, By Disease Type
Chapter 10 Multiple Sclerosis (MS) Market Segmentation, By Mode of Administration
Chapter 11 Multiple Sclerosis (MS) Market Segmentation, By Distribution Channel
Chapter 12 Regional Analysis
Chapter 13 Company profile
Chapter 14 Competitive Landscape
Chapter 15 Use Case and Best Practices
Chapter 16 Conclusion
Continued…
Check Discount on This Report @ https://www.snsinsider.com/discount/2706
About US:
SNS Insider has been a leader in data and analytics globally with its authentic consumer and market insights. The trust of our clients and business partners has always been at the center of who we are as a company. We are a business that leads the industry in innovation, and to support the success of our clients, our highly skilled engineers, consultants, and data scientists have consistently pushed the limits of the industry with innovative methodology and measuring technologies.
Contact Us:
Akash Anand – Head of Business Development & Strategy
Phone: +1-415-230-0044 (US) | +91-7798602273 (IND)


